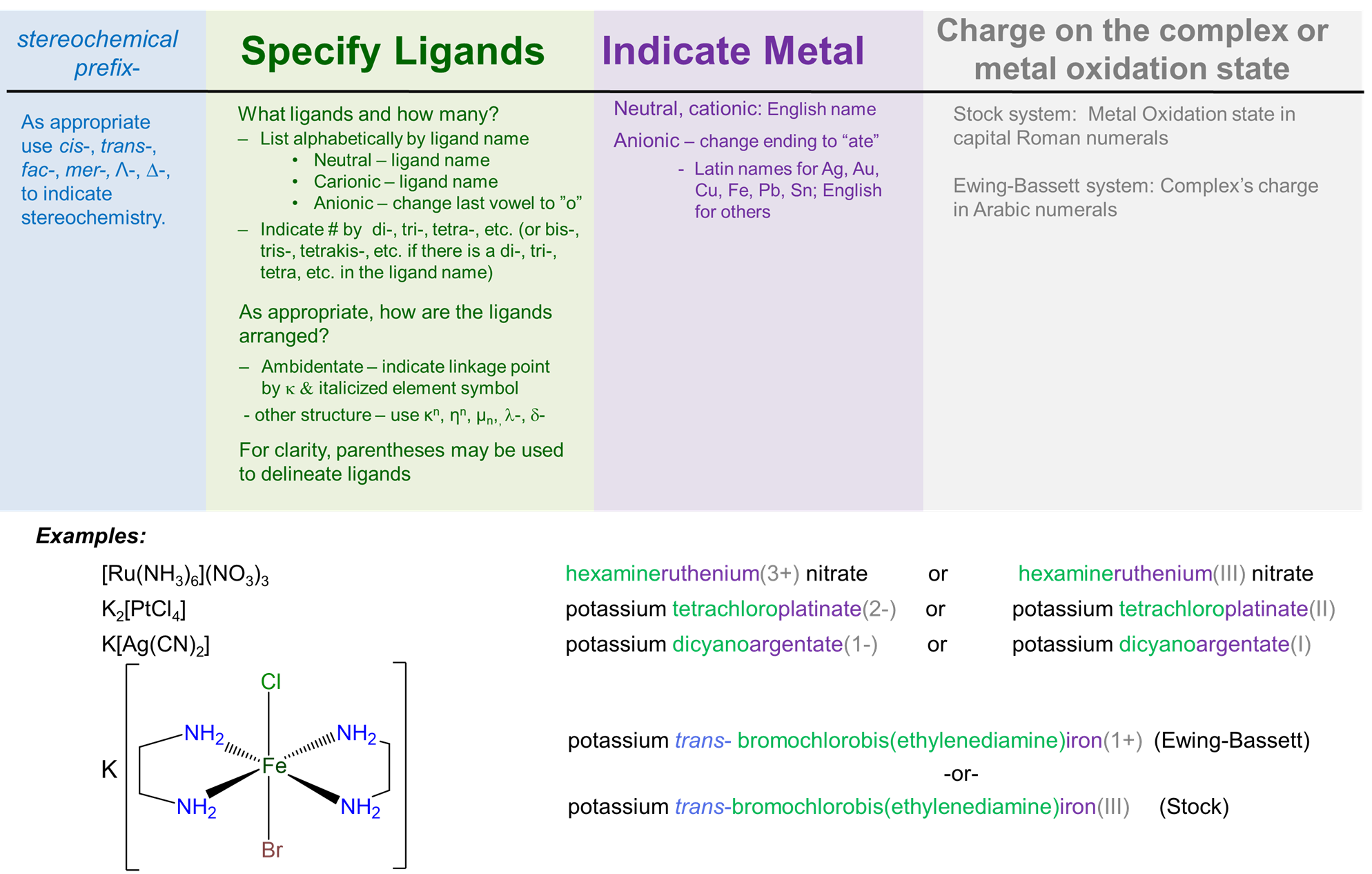
Nomenclature rules at a glance
- List ligands alphabetically by ligand name (ignore prefixes).
- Neutral ligands keep name; anionic ligands end in -o.
- Anionic complexes use metal name with -ate ending.
- Metal oxidation state in Roman numerals.
- Use cis/trans, fac/mer, kappa, and mu for structure when needed.
Naming workflow
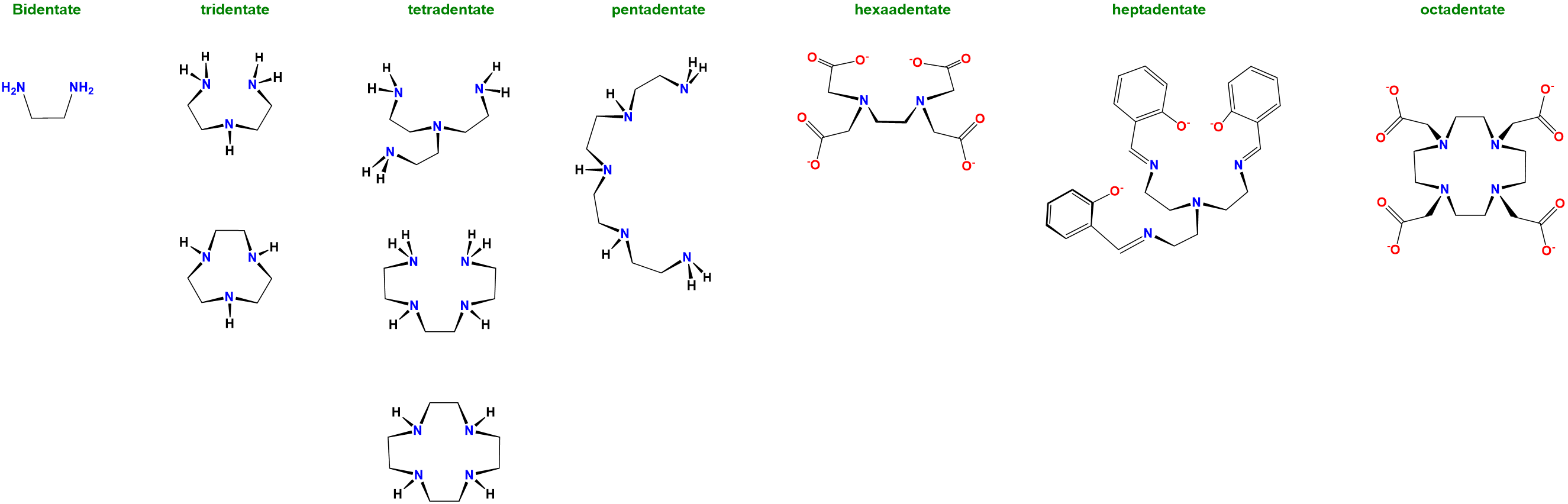
Ligand denticity
[Cr(NH3)4Cl2]Cl
Provide the full IUPAC name. Include oxidation state, ligand order, and counter ion.
3D view shows coordination geometry and ligand positions.
How the sprint works
Each prompt gives a coordination formula and a visual cue. Type the IUPAC name following ligand ordering, multiplicative prefixes, and oxidation state in parentheses. Adaptive mode queues a similar prompt after a miss.
Scoring
The grader checks ligand order, ligand identity, metal name, oxidation state, and counter ion. Small punctuation differences are forgiven; ordering mistakes are not.
Next upgrades
Add the "Build It" mode: drag ligands onto a geometry scaffold and earn points for correct coordination number and naming.