Rules and cues
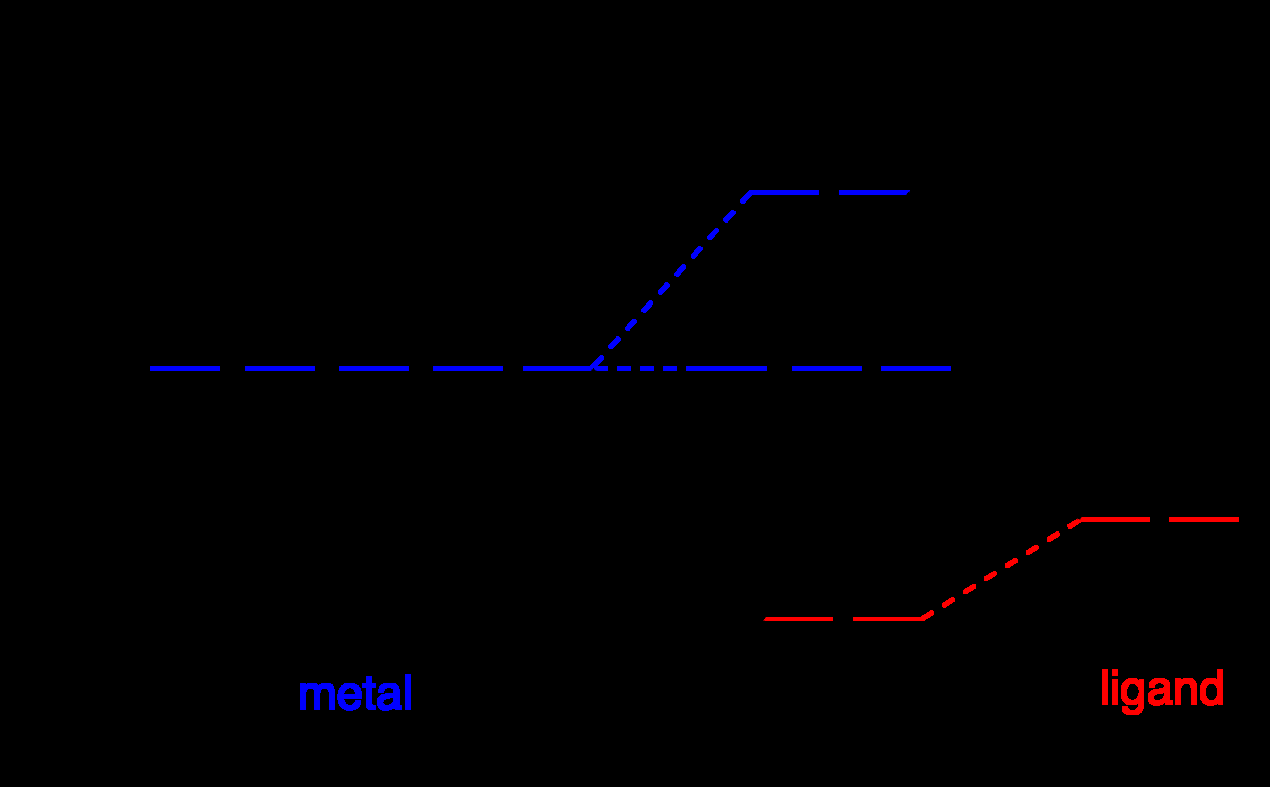
- Octahedral splitting: t2g below eg; tetrahedral is inverted.
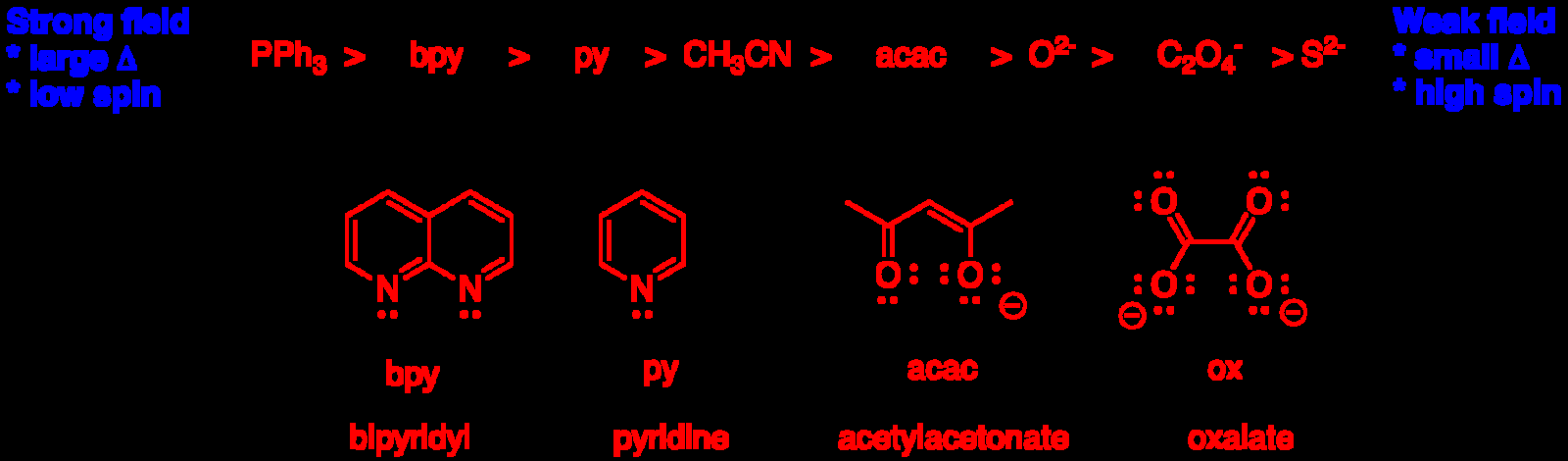
- Strong-field ligands favor low spin, large Delta.
- Square planar d8 often low spin with large splitting.
- Use spectrochemical series to rank ligand strength.
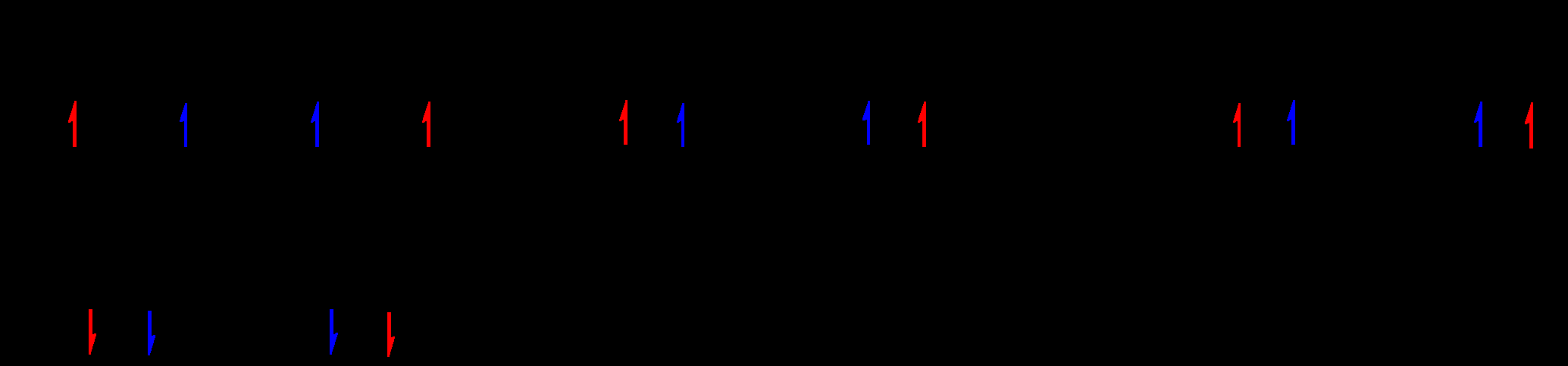
Orbital splitting (octahedral)
Ligand field overview
Spectrochemical series
Tetrahedral field

Square planar field
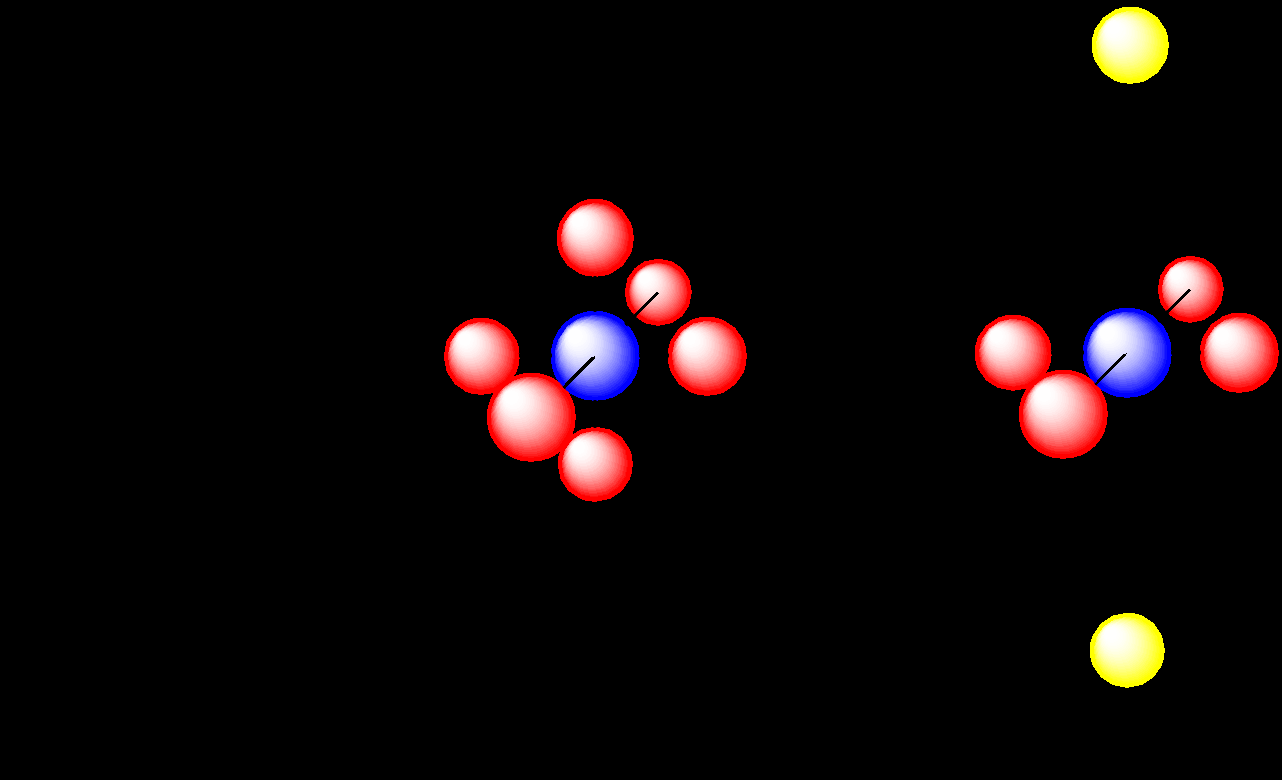
Orbital Builder
d6 octahedral
[Fe(H2O)6]2+
Place electrons and identify high/low spin.
Streak
0
Accuracy
0%
Questions
0