Coordination numbers and structures
- Coordination number equals total donor atoms bound.
- CN 2, 4, 6 dominate; 5, 7, 8, 9 are less common but important.
- Square planar vs tetrahedral depends on metal and ligand field.
- Use 3D geometry to distinguish TBP vs square pyramidal.
Geometry by coordination number
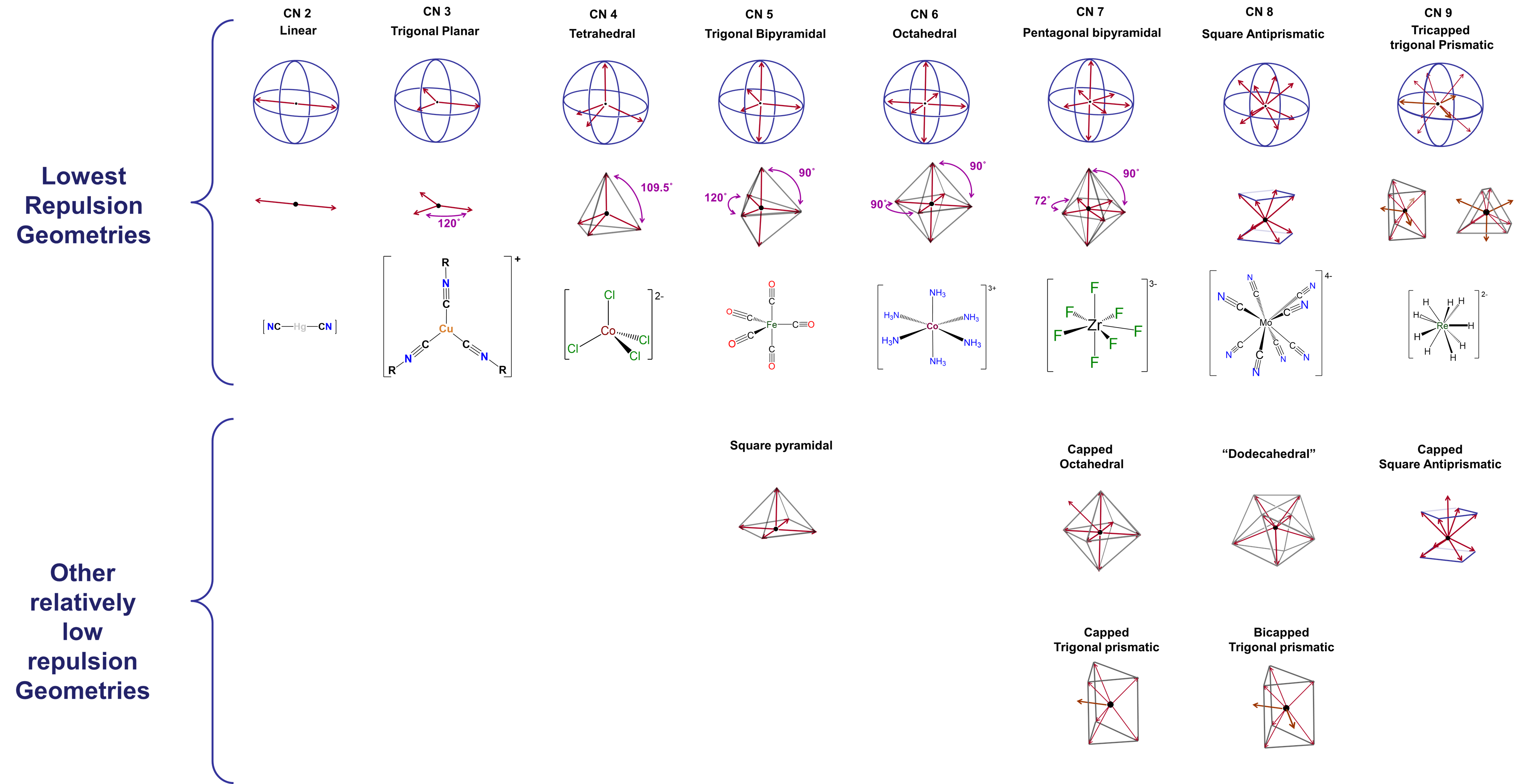
Kepert preferred geometries
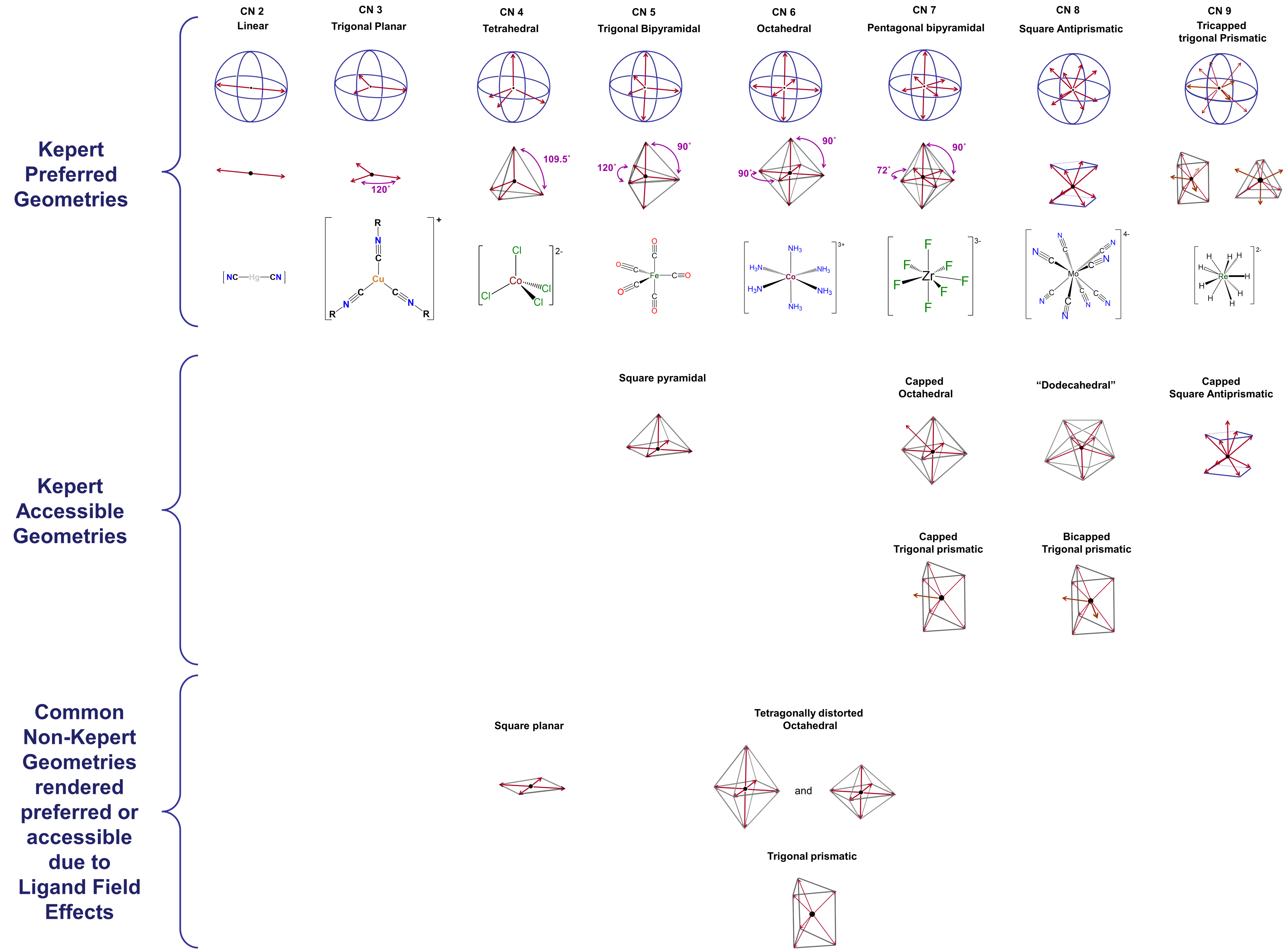
Metal preferences
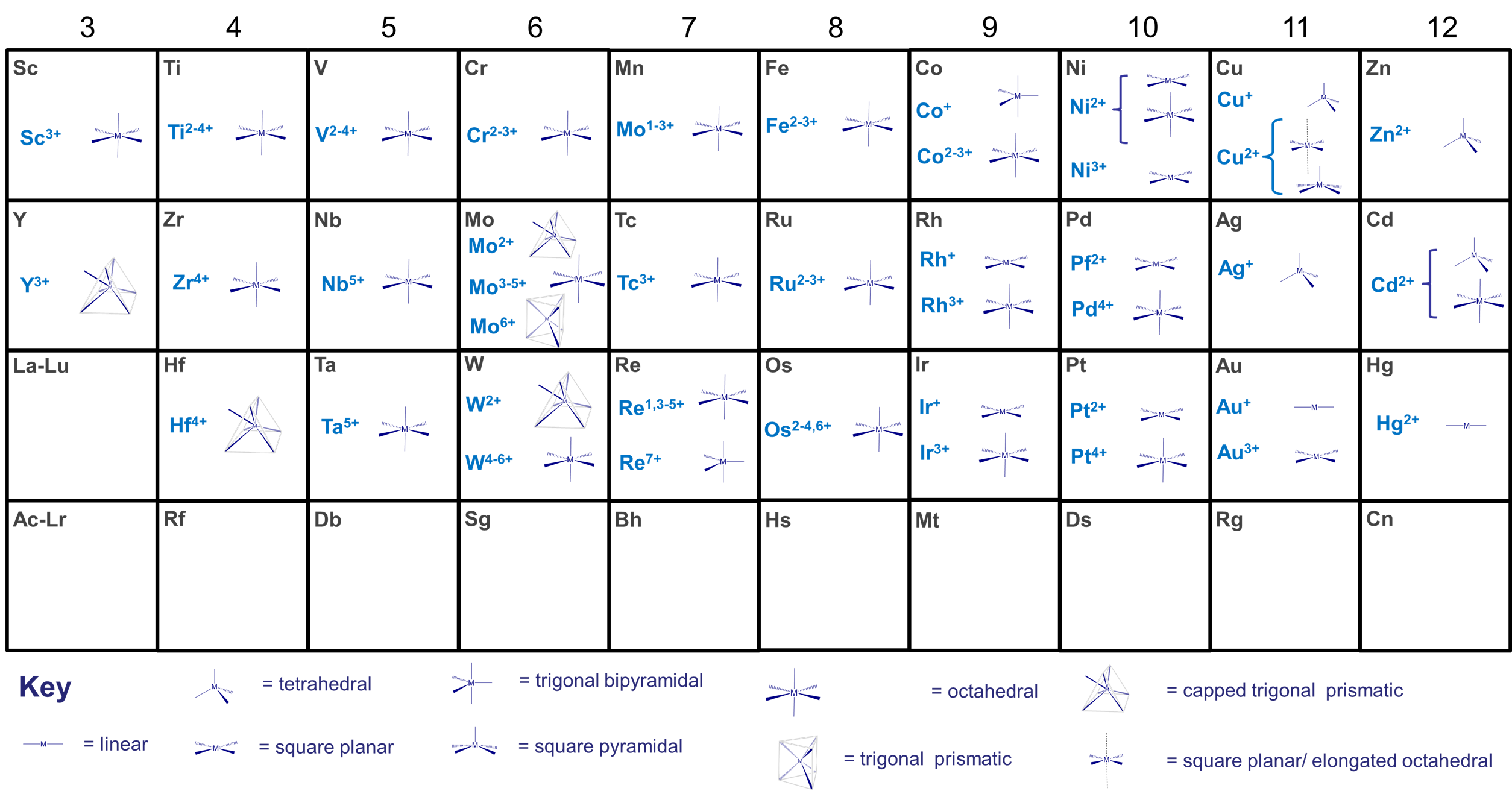
Geometry Match
CN 6
Octahedral
[Co(en)2Cl2]+
Determine coordination number and geometry.
3D model
3D view shows coordination geometry and ligand positions.
Streak
0
Accuracy
0%
Questions
0