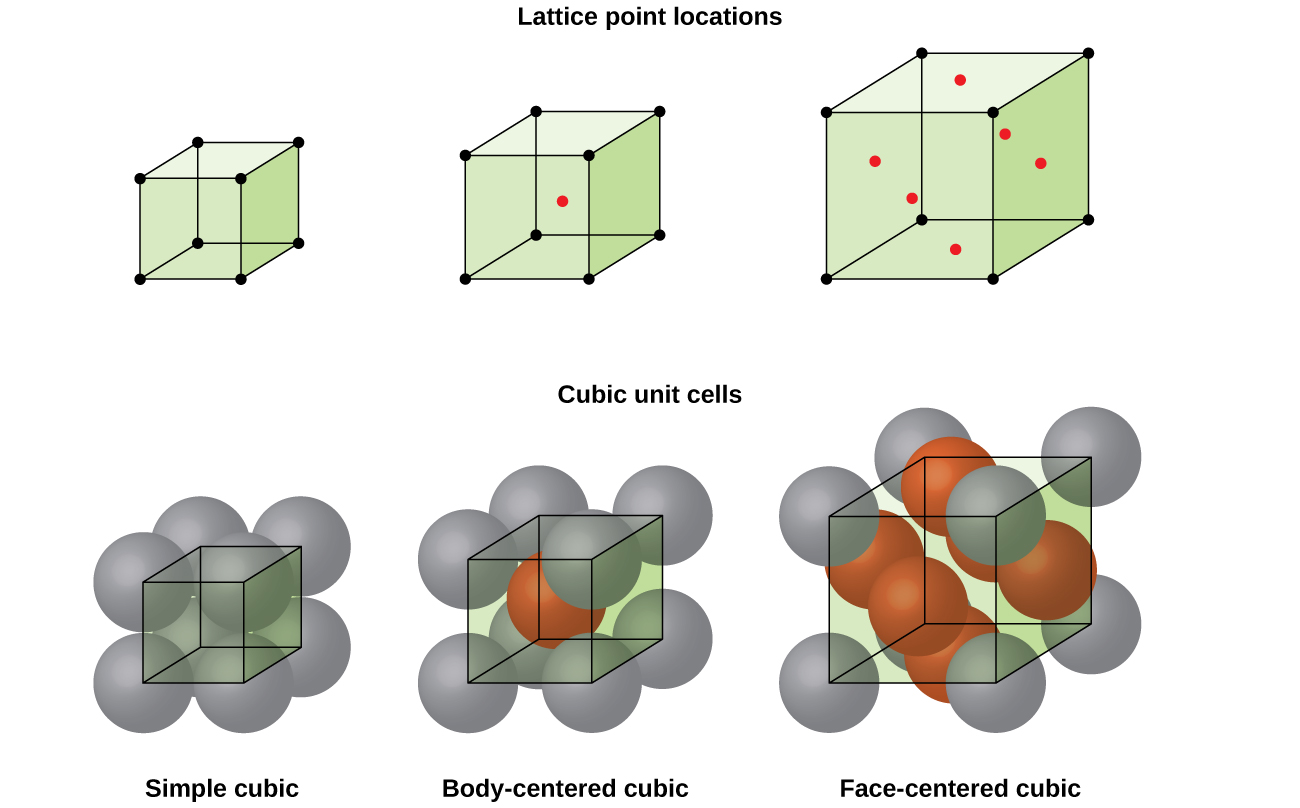
Rules of the lattice
- Simple cubic: 1 atom, CN 6, 52% packing.
- Body-centered cubic: 2 atoms, CN 8, 68% packing.
- Face-centered cubic: 4 atoms, CN 12, 74% packing.
Cubic unit cells
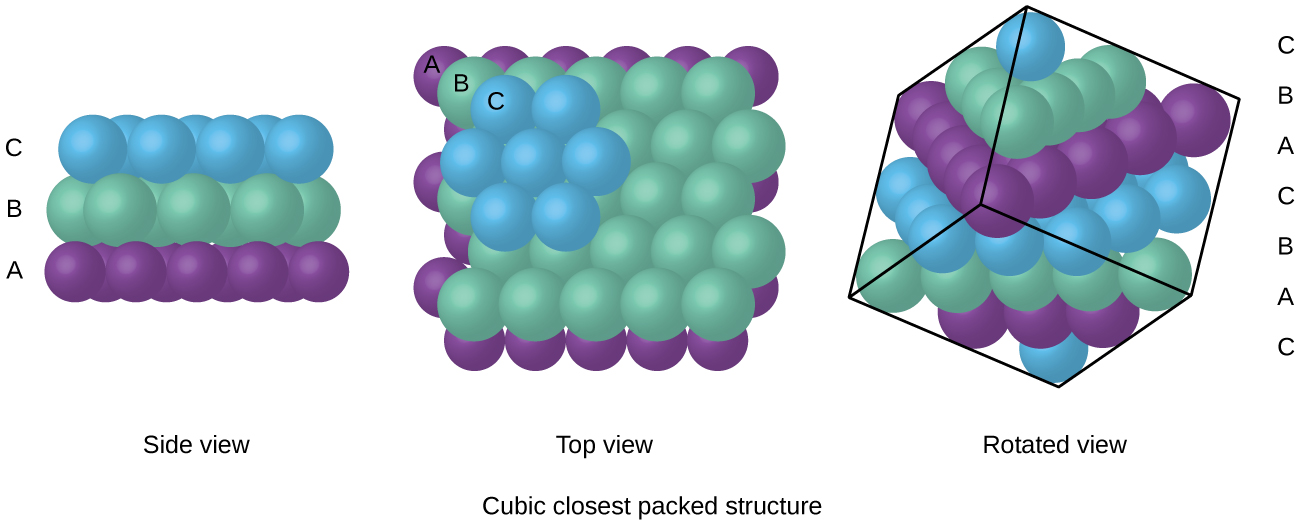
Close packing
Unit Cell ID
Cubic
Identify the unit cell
Use the figure to decide the lattice.
Streak
0
Accuracy
0%
Questions
0